Page 8 - Betesil-12pp-V3
P. 8
Clinical Studies
Ortonne Study in plaque psoriasis: BETESIL is non-inferior
versus a combination of Betamethasone 0.5 mg
- Calcipotriol 50 μg/g Ointment
(4)
A Prospective and controlled study
Multi-centre, prospective, randomised, investigator-blinded, controlled, non-inferiority trial
versus Betamethasone Dipropionate/Calcipotriol combination.
Objective
To demonstrate the non-inferiority of BETESIL once a day versus a combination of Betamethasone
0.5 mg - Calcipotriol 50 μg/g in ointment form in the treatment of chronic mild to moderate
plaque psoriasis.
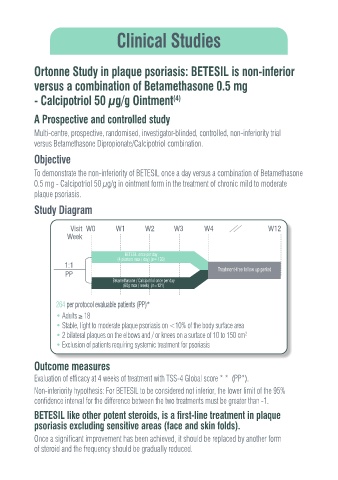
Study Diagram
Visit W0 W1 W2 W3 W4 W12
Week
BETESIL once per day
(4 plasters max / day) (n= 133)
1:1
PP Treatment-free follow up period
Betamethasone / Calcipotriol once per day
(60g max / week) (n=131)
264 per protocol evaluable patients (PP)*
• Adults ≥ 18
• Stable, light to moderate plaque psoriasis on <10% of the body surface area
• 2 bilateral plaques on the elbows and / or knees on a surface of 10 to 150 cm
2
• Exclusion of patients requiring systemic treatment for psoriasis
Outcome measures
Evaluation of efficacy at 4 weeks of treatment with TSS-4 Global score * * (PP*).
Non-inferiority hypothesis: For BETESIL to be considered not inferior, the lower limit of the 95%
confidence interval for the difference between the two treatments must be greater than -1.
BETESIL like other potent steroids, is a first-line treatment in plaque
psoriasis excluding sensitive areas (face and skin folds).
Once a significant improvement has been achieved, it should be replaced by another form
of steroid and the frequency should be gradually reduced.