Page 7 - Betesil-12pp-V3
P. 7
Clinical Studies
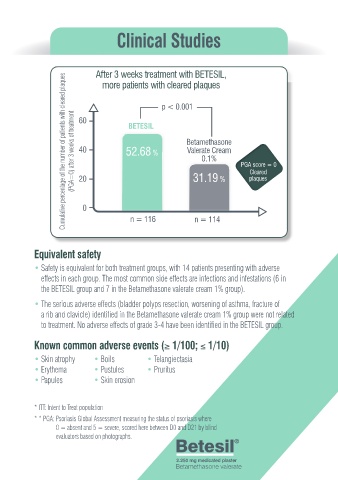
After 3 weeks treatment with BETESIL,
more patients with cleared plaques
p < 0.001
(PGA=0) after 3 weeks of treatment Cumulative percentage of the number of patients with cleared plaques 40 52.68 % Betamethasone PGA score = 0
60
BETESIL
Valerate Cream
0.1%
Cleared
31.19 %
20
plaques
0
n = 116 n = 114
Equivalent safety
• Safety is equivalent for both treatment groups, with 14 patients presenting with adverse
effects in each group. The most common side effects are infections and infestations (6 in
the BETESIL group and 7 in the Betamethasone valerate cream 1% group).
• The serious adverse effects (bladder polyps resection, worsening of asthma, fracture of
a rib and clavicle) identified in the Betamethasone valerate cream 1% group were not related
to treatment. No adverse effects of grade 3-4 have been identified in the BETESIL group.
Known common adverse events (≥ 1/100; ≤ 1/10)
• Skin atrophy • Boils • Telangiectasia
• Erythema • Pustules • Pruritus
• Papules • Skin erosion
* ITT: Intent to Treat population
* * PGA: Psoriasis Global Assessment measuring the status of psoriasis where
0 = absent and 5 = severe, scored here between D0 and D21 by blind
evaluators based on photographs.
2.250 mg medicated plaster
Betamethasone valerate